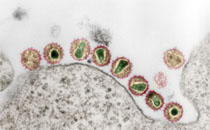
Chmielewicz B, Nitsche A, Schweiger B, Ellerbrok H (2005): Development of a PCR-Based Assay for Detection, Quantification, and Genotyping of Human Adenoviruses
Clin. Chem. 51 (8): 1365-1373, Jun 10; [Epub ahead of print].
BACKGROUND: Adenoviruses (AdVs) can cause serious disease in immunosuppressed patients, particularly those undergoing allogeneic stem cell transplantation. A method for virus quantification in clinical specimens is essential for monitoring patient adenoviral loads and evaluating new therapeutic approaches. METHODS: We developed a PCR-based assay that combines detection and genotyping of human AdVs, targeting a highly conserved region of the adenoviral genome coding for DNA polymerase (AdV DPol PCR). We tested the diagnostic applicability of this PCR-based assay by analyzing 159 clinical specimens from children with respiratory disease and comparing the results with those obtained by nested PCR analysis. RESULTS: The PCR assay detected all currently known AdV serotypes, with a detection limit of approximately 10 genome equivalents per reaction for 49 of 51 serotypes. No cross-reactivity to human DNA or other DNA viruses was observed. In addition, genotyping of PCR-positive samples was achieved within minutes by fluorescence curve melting analysis in a LightCycler instrument using 6 pairs of hybridization probes, each specific for a single AdV species. Results for clinical specimens were in good concordance with those obtained by nested PCR. CONCLUSION: The present assay is a suitable tool for the detection and genotyping of human AdVs in clinical samples.