Fabian H, Gast K, Laue M, Misselwitz R, Uchanska-Ziegler B, Ziegler A, Naumann D (2008): Early Stages of Misfolding and Association of beta2-Microglobulin: Insights from Infrared Spectroscopy and Dynamic Light Scattering
Biochemistry 47 (26): 6895-6906.
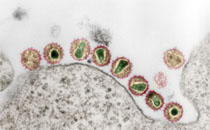
Conformational changes associated with the assembly of recombinant β2-microglobulin in vitro under acidic conditions were investigated using infrared spectroscopy and static and dynamic light scattering. In parallel, the morphology of the different aggregated species obtained under defined conditions was characterized by electron microscopy. The initial salt-induced aggregate form of β2-microglobulin, composed of small oligomers (dimers to tetramers), revealed the presence of β-strands organized in an intramolecular-like fashion. Further particle growth was accompanied by the formation of intermolecular β-sheet structure and led to short curved forms. An increase in temperature by only 25 °C was able to disaggregate these assemblies, followed by the formation of longer filamentous structures. In contrast, a rise in temperature up to 100 °C was associated with a reorganization of the short curved forms at the level of secondary structure and the state of assembly, leading to a species with a characteristic infrared spectrum different from those of all the other aggregates observed before, suggesting a unique overall structure. The infrared spectral features of this species were nearly identical to those of β2-microglobulin assemblies formed at low ionic strength with agitation, indicating the presence of fibrils, which was confirmed by electron microscopy. The observed spectroscopic changes suggest that the heat-triggered conversion of the short curved assemblies into fibrils involves a reorganization of the β-strands from an antiparallel arrangement to a parallel arrangement, with the latter being characteristic of amyloid fibrils of β2-microglobulin.